|
||
Ozone in the Atmosphere
What is ozone?
Is ozone good or bad? Thatís a trick question, because the answer is: It depends. Most ozone is found below a 30 mile (48 km) height. However, depending upon WHERE the ozone is located, it can protect or harm life. Where is good and bad ozone located?Click the image below to open the Interactive Atmosphere to see where the different parts of the atmosphere are located. The atmosphere is really a thin layer of gases surrounding the Earth. How thin? Well, imagine a basketball with one layer of aluminum foil around it. The aluminum foil represents the average thickness of the atmosphere. Thatís pretty thin! And considering without an atmosphere weíd all die, itís kind of important to take care of the atmosphere. The different layers of the atmosphereThe atmosphere is divided into regions defined primarily by temperature. The height and temperature of these layers can vary from season to season. From the Earthís surface through the bottom layer of atmosphere, called the troposphere, temperature decreases with altitude. Weather occurs in this layer. Itís also the layer we live in. The next layer up is called the stratosphere. In the stratosphere, temperature increases with altitude. This is because of ozone. When the ozone in this layer absorbs UV light from the sun, it increases in temperature. In the mesosphere, ozone concentration
decreases. This means there is less absorption of UV light in this
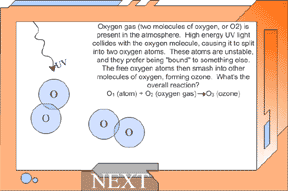
layer of atmosphere. In the upper atmosphere, called the thermosphere, temperatures are HOT. This is because incoming energy from the sun heats the molecules up. Are you wondering about the increase in temperature within the thermosphere? Well, this occurs because short-wave, high-energy solar radiation is absorbed by the (relatively few) molecules of oxygen and nitrogen. Since temperature is defined as speed of molecules, the temperature is very hot. However, since there are few molecules to transfer the heat, it would not feel hot to you because the "air" is so thin. So where is ozone "good", and where is it "bad" for life? High in the stratosphere, ozone acts as a protection from UV light. Without this protective shield, all animal life (not just humans) would be more susceptible to cancer, impaired immune systems, and eye problems like cataracts. If the ozone is close to the Earth and we breathe it in, ozone can damage our lungs. As well as harming animals, ozone also impairs the transpiration (breathing) process in plants. The amount of "good" and "bad" ozone in the atmosphere depends upon the balance between the processes that make ozone and those that destroy ozone. An upset in this balance could have serious consequences for all types of life on Earth. Ozone in the TroposphereOzone in the troposphere is "bad" for breathing, for contributing to the smog and greenhouse gases created by human activities, and it can also act as a chemical oxidant by ripping off oxygen atoms from other compounds (including you, the plants around you, other animals, etc.)! Ozone this close to the surface does not exist in high enough concentrations to shield us from UV light at this level, so its benefits are only truly seen in the stratosphere. Letís look first at how ozone is made. Oxygen gas (two molecules of oxygen, or O2) is present in the atmosphere. High energy UV light collides with the oxygen molecule, causing it to split into two oxygen atoms. These atoms are unstable, and they prefer being "bound" to something else. The free oxygen atoms then smash into other molecules of oxygen, forming ozone. Whatís the overall reaction? O1 (atom) + O2 (oxygen gas) -> O3 (ozone) The ozone is destroyed in the very process that protects us from UV rays emitted by the sun. When ozone (O3) absorbs UV light, it will split the molecule into one free oxygen atom (O1) and one molecule of oxygen gas (O2). O3 (ozone) -> O1 (atom) + O2 (oxygen gas) Ozone is valuable to us because of the way it is destroyed Ė it absorbs
UV radiation in the process. Even low-energy radiation can split ozone.
|
||